Tag Archives: Salt water Parameters

The Basics of Marine Aquarium Water Parameters
When many people are considering setting up there first marine aquarium, they can develop a perception that water chemistry (parameters) in a salt water environment is a complex topic. In my experience, this is just not the truth. While you can take this topic to a level of highly in-depth analysis and complexity, in practically every situation you will run into you can have very great success by applying a good understanding of the basics. I would like to share with you how I have learned the basics of marine aquarium water parameters. By taking this approach, the water chemistry (parameters) are not all that complicated at all.
Ammonia and Nitrites:
Ammonia and nitrites are byproducts of the nitrogen cycle at work in your aquarium. Once your aquarium is cycled, these parameters should undetectable by your test kits. Having ammonia and/or nitrite in your tank can be harmful to almost all forms of marine life. This is very similar to any fresh water aquarium. For a little more detail about the nitrogen cycle and/or cycling a marine tank, please check out the below links
https://www.reefaquarium.com/2012/the-nitrogen-cycle/
https://www.reefaquarium.com/2012/cycling-a-marine-aquarium/
Nitrates and Phosphates:
Based on what I have read, the nitrate and phosphate levels found in the ocean can vary a lot, but can be found in levels as low as 0.003ppm in most of the coral reef were we find the corals and fish that are commonly kept in the hobby. Maintaining those levels for nitrates and phosphates in an aquarium can certainly be a challenge at times, but not very difficult to do with a correctly planned and maintained set-up. I have always preferred to maintain my phosphate levels at an undetectable level by my test kit and my nitrates at the same. In the past, the nitrate level in my tank spiked at 2ppm while the tank was maturing and I was still making adjustments in my routines. Based on my experiences, the below are among the more common causes to higher levels of nitrates and phosphates:
A) Insufficient growth of nitrate eating bacteria. One of the biggest differences in the nitrogen cycle in a marine set-up when compared to a fresh water set-up is that a marine set-up with live rock will grow bacteria that will consume your nitrates and convert it into nitrogen gas. The best and most efficient way to grow enough of these bacteria is to have a good amount of live rock in your aquarium to provide a natural environment for the bacteria to grow. With enough live rock in your set-up, your nitrates should not get very high at all provide all other elements of your set-up are very well maintained. This is one of the many reasons why I always recommend the use of rock in an aquarium. The below link explains a few more benefits to having live rock in your aquarium.
https://www.reefaquarium.com/2012/what-are-the-benefits-of-live-rock/
B) Live rock adding nitrate and/or phosphates into your water. Live rock can be like a sponge and when exposed to higher levels of nitrates and/or phosphates in the water, absorbing these elements into the rock through the pours of the rock. This is very similar to osmosis. When the rock is later in tank water with lower levels of nitrates and/or phosphates than before, the rock starts to release nitrates and phosphates back into the water to restore balance between the nitrates and/or phosphate in the rock and in the water. The best way to avoid this is to cure your live rock before using it in a aquarium. The below link will explain how to cure you rock before adding it to you set-up.
https://www.reefaquarium.com/2013/curing-rock-for-marine-aquariums/
C) Over feeding adding nitrates and phosphates into the water. This can be one of the easiest problems to create for yourself, as well as one of the easiest ones to fix. Most fish foods along with the resulting fish waste, will contain both nitrates and phosphates which will affect your water parameters.
D) In efficient routine maintenance, mostly not enough water changes. You need to take a little different approach to weekly water chances on a marine tank as compared to a fresh water aquarium. Typically, most people have great success with 10% weekly water changes, however, some hobbyist have had great success with 25% biweekly water changes while other do around 5% in a effort to reduce the nitrate and/or phosphate levels in the set-up. Each set-up has different requirements so you need to find what works for your aquarium.
E) Inadequate methods to consume/remove the nitrates and phosphates. By this I am referring to methods other than waterchanges. These methods include things like: having a properly sized protein skimmer on your set-up, using chemical filter media which can remove nitrates and phosphates from the water, and using a algae scrubber to name a few of them. I have always had great results using a good quality skimmer rated for more than the actual size of my set-up, growing macro algae in a sump, and using a algae scrubber. Carbon dosing is also a option but this should be left with hobbyist that have a little more experience in order to be able to manage the risks of carbon dosing. The below links can help ad a little more detail.
https://www.reefaquarium.com/2012/setting-up-your-first-marine-aquarium-2/
Salinity
I am still amazed at how many people in the hobby today under estimate the importance of maintaining their salinity at or near 1.026, which is the average specific gravity of the ocean at the temperature and depth were we find most of the corals and fish that we keep in the hobby. The lower your salinity is from 1.026, the more you will risk having challenges maintaining other aspects of water parameters. I always prefer my salinity to be between 1.025 and 1.026. This is about 35.5 PPT (parts per thousand) for those of you who prefer using that scale/method to measure salinity. It would also be important to note that it would be well worth the expense to get yourself a good quality refactormeter to test your salinity and get accurate readings. I have found the hydrometers with the little plastic swing arms inside can (at times) be off as much as 0.003. You should avoid using one.
Alkalinity (dKH), Calcium (Cal), and Magnesium (Mag)
The alkalinity (dKH) refers to the measurement of carbonate hardness, while the calcium (Cal) and magnesium (Mag) are referring the concentration (in parts per million) of these elements in the water. This is one topic that has been found to be key to having a long term healthy reef tank and one aspect of water parameters that many hobbyist spend the majority of their efforts on. Based on the work of Randy Holmes-Farley, the below list shows what he has found to be a good balance which will help to keep the rest of your water parameters stable. I have found that I have really good success when I maintain my dKH and Cal balanced within Randy’s scale. You can find a lot more detailed information about Randy Holmes-Farley’ work at Reef Keeping Magazine (on-line magazine). I found it the easiest to keep my calcium level of 420 to 430 by keeping my alkalinity at about 9.0 to 9.6 dKH. I have also found the dKH and Cal levels will be a lot easier to maintain if you keep your Mag levels between 1280 ppm (the level of natural sea water) and 1350 ppm.
370 ppm to 1.4 dKH
380 ppm to 2.8 dKH
390 ppm to 4.2 dKH
400 ppm to 5.6 dKH
410 ppm to 7 dKH (natural seawater)
420 ppm to 8.4 dKH
430 ppm to 9.8 dKH
440 ppm to 11.2 dKH
450 ppm to 12.6 dKH
460 ppm to 14 dKH.
It is generally accepted (rule of thumb) that dKH levels between 6.5 to 7.0 and 12 with your Cal levels between 350 and 450 are considered safe. When either of those two levels ends up outside of those mentioned safe ranges, there will be some risk. Depending how far out of that range they actually are, it will mostly likely have a strong effect on your other parameters and can lead to a more serious situation. It is also interesting to note that at anytime your dKH and Mag levels drop to the lower limits within the normal/safe ranges, nuisance algaes can start to take hold.
pH
Once you have your: salinity, dKH, Cal, and Mag somewhat balanced and stable, your pH will typically stabilize between 8.0 and 8.4 which also very close to the natural range of pH you will find in different areas of the ocean. As long as yours is within the 8.0 to 8.4 range and it is stable, you will run into very few difficulties, if any at all. The below link also has some more information on pH in a marine aquarium and has some other helpful information should you run into difficulties keeping your pH stable. Another factor that can affect your pH is the quality of the water you use to mix your salt with, and the quality of your salt. I found that using reverse osmosis water with 0 TDS(total dissolved solids) and a good quality salt will really help you keep your pH stable, almost as much as the above mentioned factors.
https://www.reefaquarium.com/2012/ph-in-marine-aquariums/
Temperature
Many hobbyist seem to have a personal preference based on the requirements of either fish or corals they keep. The key is to have a stable temperature anywhere between 77F and 81F. I prefer to keep my temp between 78F and 79F.
The below chart summarizes how I approach the water parameters in my marine aquariums. It’s a very very similar approach that most people have for their fresh water aquariums with the addition of dKH, Cal and Mag levels. As you can see, there are only 4 additional elements to test for when compared to a typical freshwater se-up.
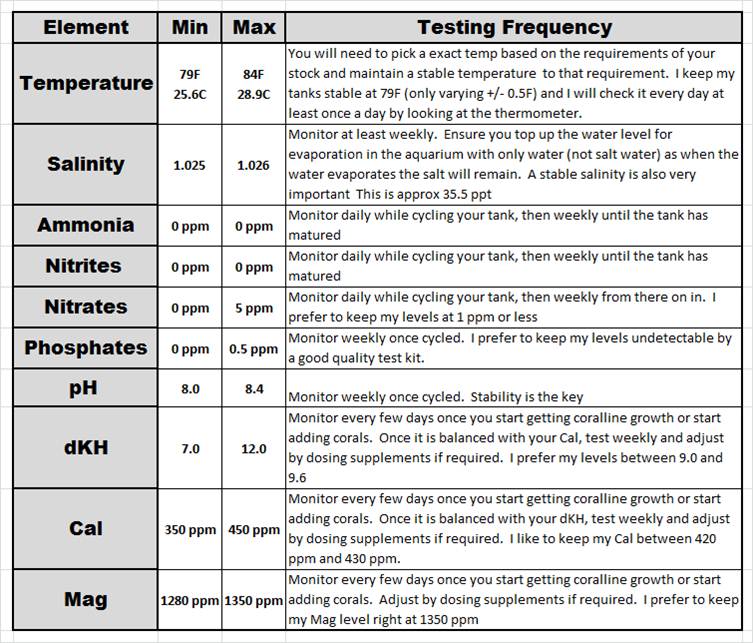
There are a lot of other aspects of marine water parameters that you can add to the above list. In my experience with the corals and fish in my set-ups, if I do a good job of maintaining those above listed parameters and remain disciplined with my weekly 10% water changes using a high quality salt with RO water, I do not have to worry about testing and maintaining anything more than this above list.
Dosing Supplements
So… Should you dose supplements to bring your parameters within the preferred range ? I have been asked many times by people new to the hobby if they should be dosing supplements. Whither or not you dose will all depend on your test results for your dKH, Cal and Mag. In my experience, lower levels of dKH, Cal, and Mag are a result of having a set-up with: higher demanding corals with classified skeletons, lots of coralline algae growth, or a combination of both.
Some low demanding systems with few (if any) corals and a disciplined weekly water changes schedule of ~10% or more, may not require any supplements to be dosed at all. This is due to the water changes replacing the used elements and maintaining your parameters which better quality salt mixes can provide you with. Aquariums with a moderate or heavy stocking of corals will typically consume a lot more of these elements out of the water than waterchanges can replace.
For those of us who keep reef tanks, these parameters are even more important to keep a close eye on as corals and coralline algae will consume more and more of these elements out of the water as they grow potentially causing changes in the water parameters. The first to be typically effected is the dKH, followed by the Cal and the last is Mag. Once/if the levels get outside of the safe range or become un-balanced with each other (even with your weekly water changes), you will need to start dosing supplements to replace the dKH and Cal which are being consumed by your corals and coralline algae. When dosing supplements you have to remember to take it slow. I would recommend getting a good quality supplement for each element (dKH, Cal, and Mag). Start by dosing at 1/8th of the recommended dosing amount while following all other of the manufacturer’s instructions printed on the bottle. After a day or two, test your parameters again and adjust your dosing amount from there. Just make sure if you are dosing all three elements on the same day, that you do not added all three at the same time. Dose only one element allowing enough time for it to become completely mixed in the water before adding the next one.
A calcium reactor can also be utilized to help maintain these three parameters. More information on calcium reactors can be found on the below link. I would not suggest people who are newer to the hobby getting and setting up a calcium reactor until you have completed a lot of research on the safe use of one.
https://www.reefaquarium.com/2012/calcium-reactors/
I prefer to either manually add the supplements to the water or to use an automatic dosing pump to add the supplements to the water. I prefer the below dosing pump.
http://www.aquaticcommunity.com/review/showproduct.php?product=500
What are good parameters for a FOWLR set-up ?
I have a different opinion of this than most other people do. You can find all kinds of information out there where people will state a different set of water parameters for fish only aquariums as fish are typically more tolerant to swings in certain parameters as compared to most corals. While this certainly is true, I am of the opinion that there is only one standard to aim for in a marine aquarium for many different reasons, some of them are described below.
A) Most of the fish we keep in fish only set-ups as well as reef aquariums come from or near the coral reefs in the ocean. While they can tolerate water parameters outside of the corals reefs, they will be a lot better off and have better long term health when kept in similar and stable parameters just as the coral reefs they are naturally found in. Fish always to better the closer we can maintain our water parameters that which we find in their natural environment (the ocean).
B) Maintaining parameters outside of the above mentioned parameters can lead to other potential problems such as a wide range of different nuisance algaes
C) Your goal should be to aim for the optimal, not the minimal. I can best make this point by using an analogy. In our modern day society, our prison systems have shown us that a human being can live out most of their natural lives in a 8’ X 8’ room, provided all other basic needs are met. Yet, when you go to find a new place for yourself to live, you don’t pick a 8’ X 8’ room. You pick a place that you can comfortably live in for many different reasons, living conditions being just one of many potential factors that you balance when making that decision. You need to take this same approach when maintaining your water parameters.
And just as a point of reference, the below chart shows the three common ways to measure alkalinity and how the values of each measurement cross reference to each other. I thought I would post this chart here as well just in case we have any readers who are used to measuring alkalinity differently than the way I am most comfortable with.
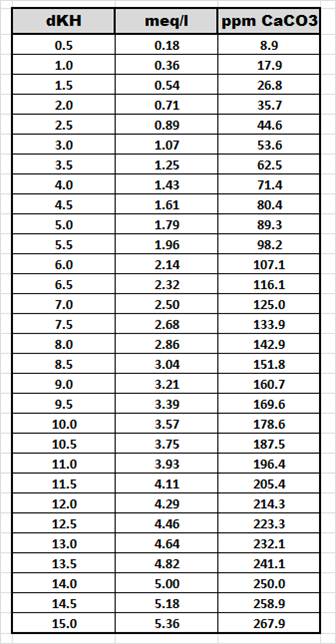
If you have any questions about anything else in this article, feel free to go to our forum and post your question using the below link. If you are not already a member, it will only take seconds to sign up.
http://www.aquaticcommunity.com/aquariumforum/
References:
http://www.windows2universe.org/earth/Water/salinity.html
http://science.nasa.gov/earth-science/oceanography/physical-ocean/salinity/
http://omp.gso.uri.edu/ompweb/doee/science/physical/chsal8.htm
http://ocean.nationalgeographic.com/ocean/critical-issues-ocean-acidification/
http://www.ocean-acidification.net/FAQacidity.html
http://www.reefkeeping.com/issues/2004-05/rhf/index.php
http://www.reefkeeping.com/issues/2006-06/rhf/index.php